Shanghai, China, May 8, 2020 – Shanghai Henlius Biotech, Inc. (2696.HK) announced that Henlius has received approval notification for the clinical trial application of HLX56, an innovative, humanised anti-DR4 (death receptor 4) monoclonal antibody (mAb) injection in Taiwan China, and the approval indication is soild tumour.
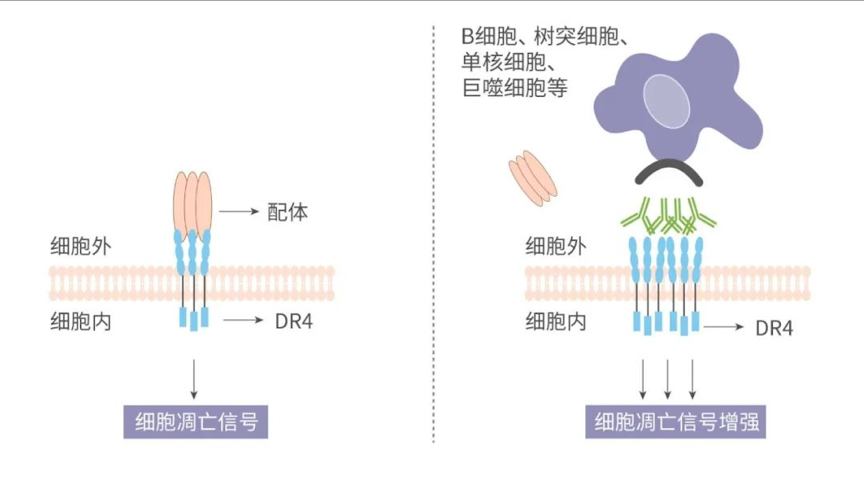
HLX56 is an innovative anti-DR4 mAb, which can specifically bind to DR4 expressed on the surface of tumour cells, and induce tumour cell apoptosis. DR4 is a member of the TNFR (tumour necrosis factor receptor) superfamily. Studies have shown that DR4 is expressed in a variety of solid tumours including colorectal, pancreatic, ovarian, gastric, uterine, lung and breast cancers[1]. Binding of DR4 to its ligand, TRAIL (TNF-related apoptosis-inducing ligand), can induce the trimerisation of DR4, which will activate the downstream pathway and lead to apoptosis of tumour cells.
HLX56 is a DR4 agonist antibody with optimized Fc. Binding of HLX56 to DR4 can also promote DR4 oligomerisation and activation and subsequent apoptosis of tumour cells. It is noteworthy that two mutations were introduced into the Fc regions of HLX56 to increase affinity to the Fc gamma receptor IIb (FcγRIIB) on human peripheral blood mononuclear cells (PBMC). Crosslinking provided by FcγRIIB can significantly enhance the oligomerisation and activation of DR4, and finally amplifies the apoptotic signal. Meanwhile, this special design also improves the safety of HLX56.
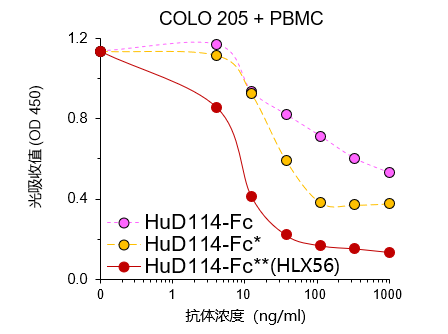
HLX56 has demonstrated potent anti-tumour effect and good safety in in vivo and in vitro preclinical studies. In human colon cancer cell lines, low dose of HLX56 demonstrated strong anti-tumour effect in the presence of human PBMC, which implies that HLX56 may overcome the problem of low potency in clinical studies of previous DR4 agonists.
New, highly potent antibodies to death receptors having Fc mutations to increase antitumor activity.[2]
So far, there are none DR4 targeted drugs marketed in the world. Considering its preclinical performance, the clinical study of HLX56 is expected to move forward quickly and smoothly. HLX56 also has the potential to be the first-in-class drug targeting DR4. HLX56 has the potential to be used in the treatment of cancers with high DR4 expression, such as colorectal cancer, non-small cell lung cancer and cervical cancer, and the development of HLX56 will further expand the coverage of Henlius pipeline. In addition, the mechanism of DR4 activation-induced apoptosis is distinguished from that of other mainstream treatments for solid tumours, such as chemotherapy, anti-angiogenesis therapy, immuno-oncology therapy, etc., and the toxicity of DR4 agonist on normal cells is relatively low. These characteristics make DR4 agonist very suitable for combination therapies. In the future, HLX56 is expected to be evaluated in combination therapies with other anti-tumour drugs on Henlius pipeline. Besides, the R&D progress of HLX56 will also help Henlius to accumulate relevant data and experience on pathways involved in death receptor-induced apoptosis.
Leveraging the established and integrated bispecific antibody R&D platform, this experience will provide valuable insights for the discovery and development of bispecific antibodies with DR4 as one of the targets.
Looking forward, Henlius will continue advancing the discovery and development of bio-innovatives based on its established and integrated innovation platform, underscoring its long-term commitment to providing affordable and effective therapies for patients worldwide.
Reference
1. Halpern W, Lincoln C, Sharifi A, et al. Variable distribution of TRAIL receptor 1 in primary human tumor and normal tissues. Eur J Cancer 2004; (Suppl 2; abstr 225).
2. Wang L, Ding Y, Park H, et al. Abstract 3493: New, highly potent antibodies to death receptors having Fc mutations to increase antitumor activity. Cancer Research, 2016, 76; (Suppl 14):3493-3493.
