Led by a quality management team with extensive international experience and underpinned by quality-centric culture, Henlius has established exceptional systems for quality control and effeciency.
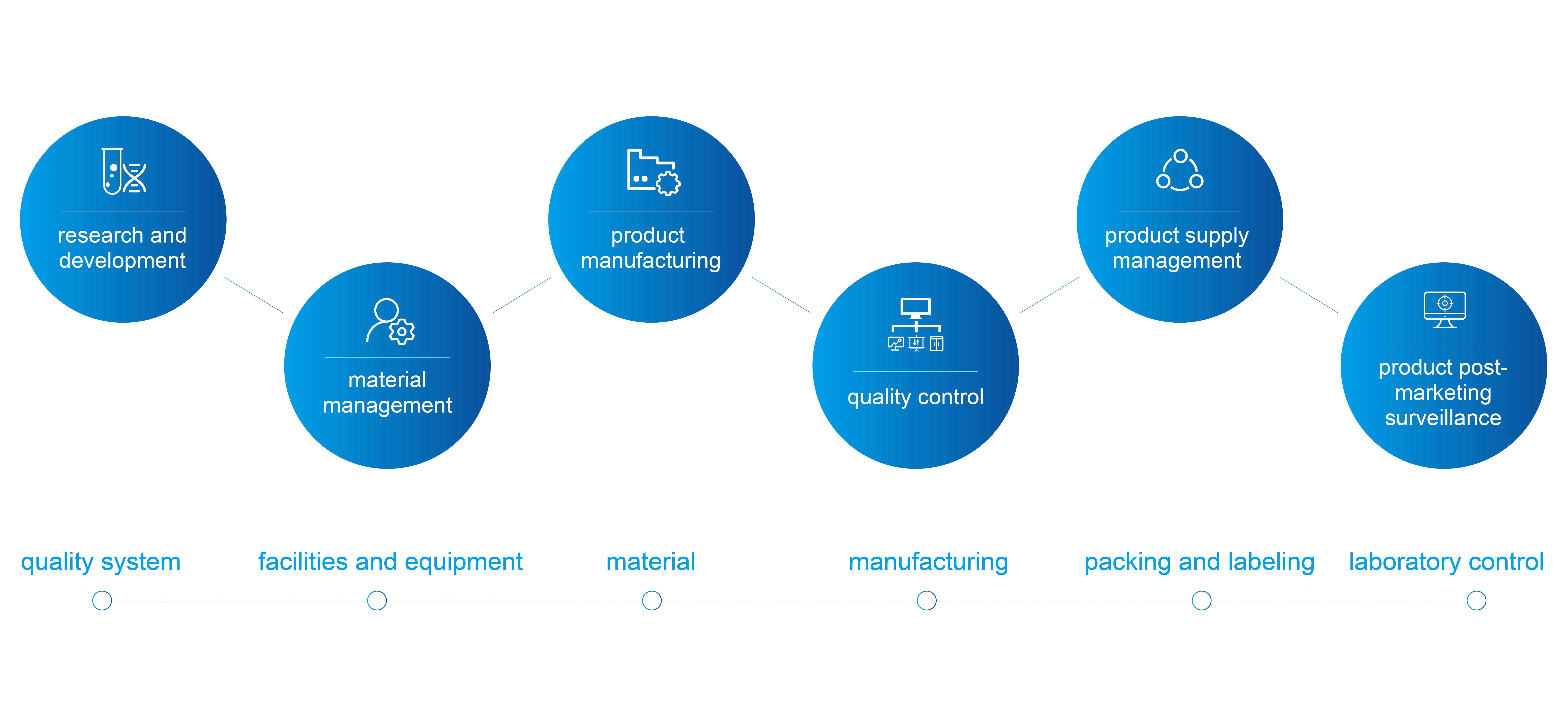
The Henlius quality management system is in line with international quality standards for the entire product life cycle, from research and development to material management, product manufacturing, quality control, product supply management, and product post-marketing surveillance.
The manufacturing sites and their quality management systems have passed nearly 120 on-site inspections and audits conducted by international agencies including the EMA, the FDA, the EU Qualified Person, as well as Henlius’ international business partners, and have been GMP-certificated by China, the EU and the US regulatory agencies as well as various PIC/S participating members (Brazil and Indonesia).
