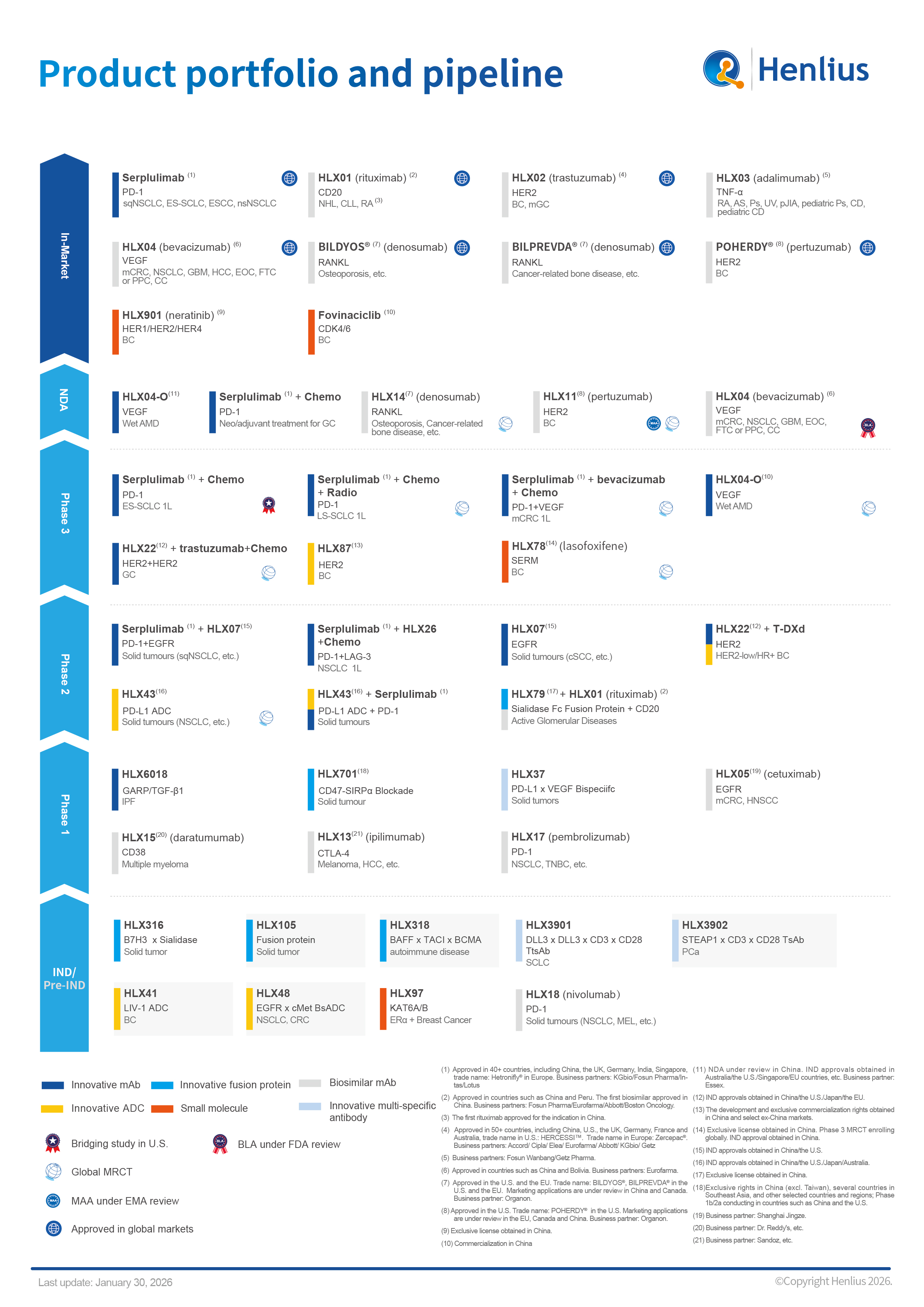
At present, Henlius has 10 products have been approved for marketing worldwide, and 6 marketing applications under review in China, the U.S. and the EU, respectively. Meanwhile, Henlius has conducted over 30 clinical studies for 19 products globally. To date, Henlius has launched products include HANSIZHUANG (serplulimab, trade name: Hetronifly® in Europe), the world’s first anti-PD-1 mAb for the first-line treatment of SCLC, HANQUYOU (trastuzumab, trade name: HERCESSI™ in the U.S., Zercepac® in Europe), a China-developed mAb biosimilar approved in China, Europe and U.S., HANLIKANG (rituximab), the first China-developed biosimilar, denosumab Bildyos® and Bilprevda®, and pertuzumab Poherdy®.