Shanghai, China, June, 30th, 2022 - Shanghai Henlius Biotech, Inc. (2696.HK) announced that the investigational new drug application (IND) of HLX53, an anti-TIGIT Fc fusion protein, in patients with advanced/metastatic solid tumors or lymphomas has been approved by the National Medical Products Administration (NMPA). At present, there is no anti-TIGIT has been approved for marketing globally.
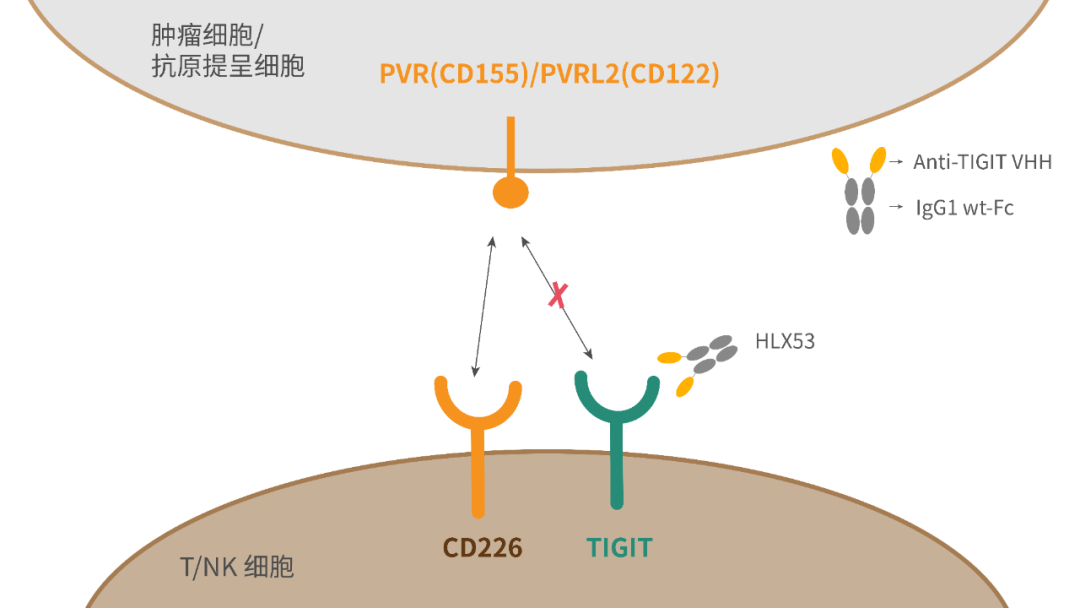
T-cell immunoglobulin and ITIM domains (TIGIT) has emerged as popular inhibitory checkpoint receptor, which is mainly expressed on natural killer (NK) cells, activated CD8+ T and CD4+ T cells, and T regulatory cells. TIGIT binds to the ligand CD155 (poliovirus receptor, PVR), mainly expressed on antigen-presenting cells (APC) or the surface of tumour cells, to down-regulate T cell and NK cell functions1-2. As an immune checkpoint protein, TIGIT can inhibit innate and adaptive responses in various mechanisms of action and act as “brakes” like PD-1/PD-L1 does to stop T cells from attacking tumours3. Studies have shown that TIGIT inhibitor are effective against lung cancer, gastric cancer, melanoma, multiple myeloma, etc. HLX53 is an anti-TIGIT Fc fusion protein independently developed by Henlius, consisting of variable domain of heavy chain of heavy-chain antibody (VHH) and wildtype IgG1 Fc. Pre-clinical studies have demonstrated that HLX53 exhibits excellent tumor inhibition4 with good safety.
Underpinned by the patient-centric strategy, Henlius has built an innovative product pipeline with many emerging targets, including PD-1/L1, LAG-3, TIGIT, BRAF, etc. The company has also independently developed HLX301, a recombinant humanized anti-PDL1 and anti-TIGIT bispecific antibody (BsAb), in patients with advanced tumours. Currently, the first patient has been dosed in Australia in Phase 1 clinical trial of HLX301 and the IND application has been approved in China for the treatment of advanced tumors. Ahead of the same class of BsAb targeting PD-L1×TIGIT, HLX301 is potentially to be the first-in-class anti-PD-L1×TIGIT BsAb. Looking forward, Henlius will continue conducting clinical studies for more innovative products in bispecific antibodies and the antibody-drug conjugates (ADC) and exploring combination therapies with improved efficacy to provide patients with quality and affordable biologics.
References:
[1] Chauvin JM, Zarour HM. TIGIT in cancer immunotherapy. J Immunother Cancer. 2020;8(2).
[2] Sanchez-Correa B, Valhondo I, Hassouneh F, et al. DNAM-1 and the TIGIT/PVRIG/TACTILE Axis: Novel Immune Checkpoints for Natural Killer Cell-Based Cancer Immunotherapy. Cancers (Basel). 2019;11(6):877.
[3] Yue C, Gao S, Li S, Xing Z, Qian H, Hu Y, Wang W and Hua C (2022) TIGIT as a Promising Therapeutic Target in Autoimmune Diseases. Front. Immunol. 13:911919.
[4] Botong Hua, Ming Yang, Jie Xue, Chen Dong, Yi-Ting Mao, Ou Li, Eric Cheung, Hassan Issafras, Wenfeng Xu, Weidong Jiang; Abstract 2451: A novel single domain antibody targeting TIGIT for cancer use in combination therapies. Cancer Res 1 July 2021; 81 (13_Supplement): 2451.
