Shanghai, China, June 6th, 2023 –Shanghai Henlius Biotech, Inc. (2696.HK) announced that the results from the phase 2 study (HLX208-LCH/ECD201) of HLX208, the company’s innovative BRAFV600E inhibitor, in adult Langerhans cell histiocytosis (LCH) and Erdheim-Chester disease (ECD) with BRAFV600E mutation were released as a poster presentation at 2023 American Society of Clinical Oncology (ASCO) Annual Meeting. HLX208-LCH/ECD201 study was led by Professor Jian Li from Peking Union Medical College Hospital, Chinese Academy of Medical Sciences & Peking Union Medical College. In April 2023, the Center for Drug Evaluation (CDE) of China National Medical Products Administration (NMPA) has granted a Breakthrough Therapy Designation (BTD) to HLX208 for the treatment of adult LCH and ECD with BRAFV600E mutation.
ECD and LCH are currently regarded as myeloid neoplasias with inflammatory properties, significantly affecting patients’ quality of life. The National Health Commission (NHC) has placed them on the "First National List of Rare Diseases"[1]. According to the 2019 edition of the Clinical Practice Guidelines for Rare Diseases issued by the NHC of China[2], LCH and ECD are driven by constitutive activation of the MAPK/ERK signalling pathway with more than 50% incidence of BRAFV600E mutation. HLX208 is a potential “best-in-class” BRAF inhibitor introduced from NeuPharma with a proprietary novel chemical structure that differs from other marketed BRAF inhibitors. It exhibited a single crystal morph, high bioavailability, and excellent anti-tumour efficacy in preclinical studies. Subsequent early clinical data also demonstrated preliminary efficacy and good safety and tolerability in patients with cancer, warranting further clinical development. The data released at 2023 ASCO are as follows:
Title
A phase 2 study of HLX208, a BRAFV600E inhibitor, in adult patients with Langerhans cell histiocytosis and/or Erdheim-Chester disease harboring BRAFV600E mutation. (Abstract No. 7574)
Study design
This was a single-arm, open-label, multicenter, phase 2 study. Patients with histologically confirmed Langerhans cell histiocytosis (LCH) and/or Erdheim-Chester disease (ECD) were enrolled and received oral administration of HLX208 at 450 mg twice every day. HLX208 was given in 28-day cycles until disease progression, experiencing intolerable toxicity, withdrawal of consent, initiation of new antitumor therapy, or death, whichever occurred first. The primary endpoint was the objective response rate (ORR) assessed by independent review committee (IRC) per PET Response Criteria in Solid Tumors (PERCIST) 1.0. Secondary endpoints included safety, other efficacy measures, and pharmacokinetics of HLX208.
Results
As of January 15, 2023, 22 patients with LCH and/or ECD were enrolled. All patients have received at least one dose of HLX208 and were included in the current analysis. The median age was 39 (range 18–69) years. Nine (40.9%) patients were male. 12 (54.5%) patients were diagnosed with LCH, 9 (40.9%) were diagnosed with ECD, and 1 (4.5%) had combined LCH and ECD. 6 (27.3%) patients had single system multifocal disease, while 16 (72.7%) had multisystem disease. The median follow-up duration was 4.8 months (95% CI 2.7–5.7).
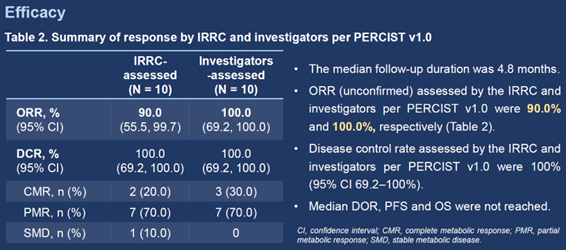
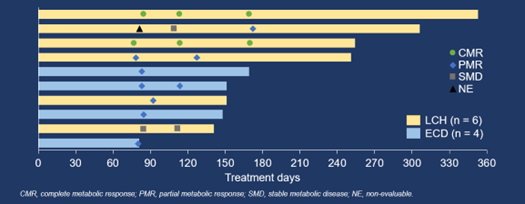
Among the 10 efficacy evaluable patients, ORR (unconfirmed) assessed by IRC per PERCIST 1.0 was 90.0% (95% CI 55.5–99.7%) (9/10, including 2 complete metabolic response [CMR] and 7 partial metabolic response [PMR]), while that by investigators per PERCIST 1.0 was 100.0% (95% CI 69.2–100.0%) (10/10, including 3 CMR and 7 PMR). Disease control rate was 100.0% (95% CI 69.2–100.0%) as assessed by both IRC and investigators per PERCIST 1.0. The median duration of response, median progression-free survival, and median overall survival were not reached.
Of the 22 patients who received the drug, 17 (77.3%) patients experienced treatment-emergent adverse events (TEAEs); 12 (54.5%) reported treatment-related adverse events (TRAEs), most commonly alanine aminotransferase increased (36.4%), aspartate aminotransferase increased (22.7%), γ-glutamyltransferase increased (18.2%), and blood lactate dehydrogenase increased (18.2%). Most TRAEs were grade 1 (27.3%) and 2 (18.2%). There were no TEAEs that led to treatment discontinuation or death.
Conclusion
In summary, HLX208 was safe and well tolerated, and showed promising efficacy in patients with LCH and/or ECD.
