Shanghai, China, June 6th, 2023 –Shanghai Henlius Biotech, Inc. (2696.HK) announced that the results from the phase 2 study (HLX07-ESCC201) of HLX07, the company’s innovative humanised anti-epidermal growth factor receptor (EGFR) monoclonal antibody, in patients with locally advanced, unresectable, or metastatic esophageal squamous cell carcinoma (ESCC) were released as a poster presentation at 2023 American Society of Clinical Oncology (ASCO) Annual Meeting for the first time. The study was led by Professor Jing Huang from the National Cancer Center/National Clinical Research Center for Cancer/Cancer Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College.
HLX07 is an innovative drug targeting EGFR independently developed by Henlius. Adopting the self-developed advanced antibody engineering platform, Henlius re-engineered cetuximab by humanising its Fab regions and minimizing its glycan contents to generate HLX07 to reduce the immunogenicity and maintain high affinity of the product. As of now, Henlius holds patents of HLX07 in several jurisdictions including China, the United States, the European Union, Australia, and Japan. Currently, Henlius is conducting phase 2 clinical trials to explore HLX07 as monotherapy or in combination with HANSIZHUANG (serplulimab) for the treatment of solid tumours including ESCC and cutaneous squamous cell carcinoma (CSCC). The data of HLX07-ESCC201 study released at 2023 ASCO Annual Meeting are as follows:
Title
A phase 2 study of HLX07 as monotherapy or combination therapy in patients with locally advanced, unresectable, or metastatic esophageal squamous cell carcinoma. (Abstract No. 4029)
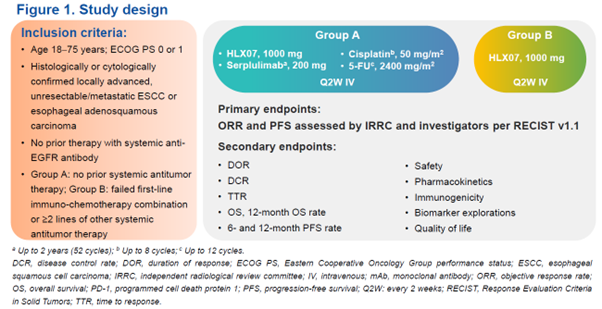
Study design
In this open-label, multicenter phase 2 study, patients aged 18–75 years with histologically or cytologically confirmed locally advanced, unresectable/metastatic esophageal squamous cell carcinoma (ESCC) or esophageal adenosquamous carcinoma were enrolled. Patients with no prior systemic antitumor therapy were assigned to group A and given HLX07 1000 mg (anti-EGFR monoclonal antibody) plus serplulimab 200 mg (anti-PD-1 monoclonal antibody) and chemotherapy (5-FU 2400 mg/m2 + cisplatin 50 mg/m2), Q2W IV. Patients who had failed first-line immuno-chemotherapy combination or at least two lines of other systemic antitumor therapy were assigned to group B and given HLX07 monotherapy (1000 mg Q2W IV). The primary endpoints were objective response rate (ORR) and progression-free survival (PFS) assessed by an independent radiological review committee and investigators per RECIST v1.1. Secondary endpoints included other efficacy measures, safety, pharmacokinetics, immunogenicity, and biomarker explorations.
Results
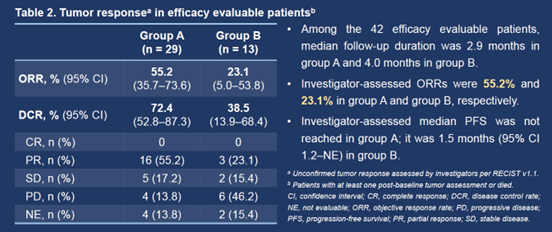
As of February 4, 2023, 49 patients were enrolled in group A (n = 30) and group B (n = 19), with a median age of 64.5 and 59.0 years, respectively. 26 (86.7%) patients in group A and all patients in group B were male. The median follow-up duration was 2.9 months, and the preliminary efficacy was presented.
Among the 42 efficacy evaluable patients (29 in group A and 13 in group B), investigator-assessed ORRs were 55.2% (95% CI 35.7–73.6%) and 23.1% (95% CI 5.0–53.8%) in the respective groups. Investigator-assessed median PFSs were not reached in group A and 1.5 months (95% CI 1.2–not evaluable) in group B.
Most HLX07-related adverse events (AEs) were of grade 1 or 2. AEs of special interest were observed in 16 (53.3%) and 11 (57.9%) patients in group A and group B, respectively, most commonly rash (43.3% vs 47.4%) and hypomagnesemia (33.3% vs 36.8%). No drug-related death was reported.
Conclusion
In summary, HLX07 showed encouraging antitumor activity with a manageable safety profile in patients with advanced ESCC.
