Shanghai, China, April 10th, 2024 – Shanghai Henlius Biotech, Inc. (2696. HK) announced that the exploratory biomarker analysis of ASTRUM-005, Henlius’ phase 3 clinical study of its innovative anti-PD-1 monoclonal antibody(mAb) HANSIZHUANG (serplulimab) as first-line treatment for patients with extensive-stage small cell lung cancer (ES-SCLC) was published as poster presentation at the American Association for Cancer Research 2024 meeting (AACR 2024), with Professor Ying Cheng from Jilin Province Cancer Hospital as the leading principal investigator of this study.
HANSIZHUANG (serplulimab) is the company’s first innovative product, as well as the world's first anti-PD-1 mAb for the first-line treatment of SCLC. As of now, HANSIZHUANG has been approved in China and Indonesia, making it also the first China anti-PD-1 mAb approved in Southeast Asia. Since its first approved in China in March 2022, HANSIZHUANG has been approved for 4 indications in China including MSI-H solid tumour, squamous non-small cell lung cancer (sqNSCLC), ES-SCLC, and esophageal squamous cell carcinoma (ESCC), benefiting more than 60,000 patients. The marketing applications of the first-line treatment for non-squamous non-small cell lung cancer (nsNSCLC) and ES-SCLC are under review by the NMPA and the European Medicines Agency (EMA), respectively. Furthermore, the company submitted marketing applications for HANSIZHUANG in Thailand, Singapore, Malaysia to further promote the product in Southeast Asia. Moreover, Henlius also launched a head-to-head bridging trial of HANSIZHUANG versus first-line standard-of-care atezolizumab for ES-SCLC in the U.S. and the company plans to submit a Biologics License Application (BLA) for HANSIZHUANG in the U.S. in 2024.
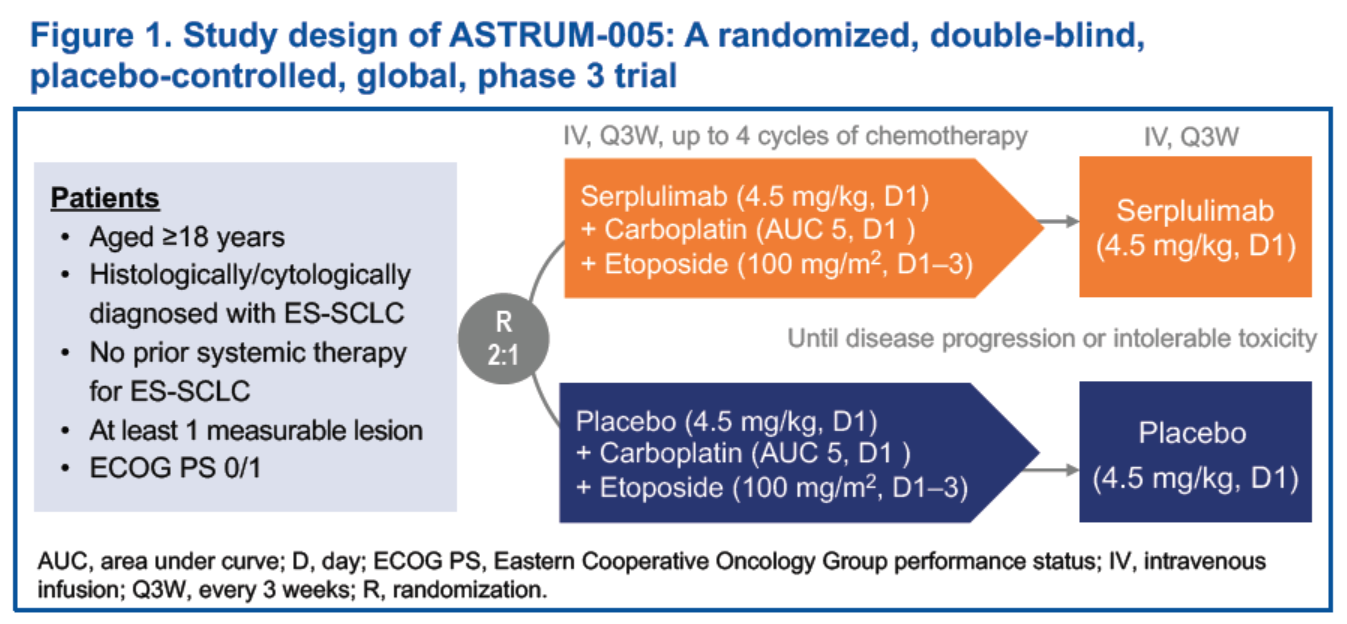
The approval of HANSIZHUANG for the treatment of ES-SCLC was primarily based on ASTRUM-005 (NCT04063163), a randomised, double-blind, international, multicentre, phase 3 study aimed to compare the efficacy and safety of serplulimab, versus placebo in combination with chemotherapy in previously untreated ES-SCLC patients. The results of ASTRUM-005 were first presented at 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, and published in the Journal of the American Medical Association (JAMA), one of the top four medical journals in the world in September 2022. Thereafter, the updated results of ASTRUM-005 were released at the 2022 European Society for Medical Oncology Asia (ESMO Asia) Congress. The results of ASTRUM-005 has showed that a PD-L1 tumor proportion score (TPS)≥ 1% did not appear to be associated with better response to serplulimab plus chemotherapy. To elaborate biological characteristics of the ES-SCLC patients who has benefited from serplulimab in this study, Henlius completed an exploratory biomarker analysis to retrospectively evaluate the association of proteome signature, genetic mutations, and hematological indices with the efficacy of serplulimab.
Details of the results presented at 2024 AACR Annual Meeting are as follows:
Title
Exploratory biomarker analysis of phase 3 ASTRUM-005 study: Serplulimab versus placebo plus chemotherapy for extensive-stage small cell lung cancer
Methods
Biomarker Analysis of the ASTRUM-005 Study
Study design of ASTRUM-005 is presented in Figure 1.
Correlation analysis between the proteomic, genomic and hematological parameters of the patients and the treatment efficacy were conducted in this study. Proteomic and genomic assays were performed in the central laboratory. Serum samples were collected from patients and serum proteomics data were generated via the Olink® Explore 3072 platform. While tumour DNA was extracted from tumour samples and genomic mutations were assessed by a Med1CDxTM panel. Hematology tests were performed at hospital sites, with hematologic parameters of neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR) and lactate dehydrogenase (LDH) levels were included in the analysis.
Statistical Analysis
For the analysis of objective response rate (ORR), 95% confidence interval (CI) of rate was calculated by the Clopper-Pearson method, and the odds ratio and its 95% CI were estimated by Cochran-Mantel-Haenszel statistics. And for progression-free survival (PFS) and OS, the median was calculated from product-limit (Kaplan-Meier) estimates, while n was the number of patients in each subgroup category. The hazard ratio and its 95% CI were estimated using an unstratified Cox proportional hazards model. Efron's method was used to handle ties.
For serum proteomics, differentially expressed proteins (DEP) were identified using a t-test between responders (patients showing complete response or partial response) and non-responders (patients with stable disease or progressive disease) in the treatment group. Predictive/prognostic biomarkers are identified through generalized linear model selection with 5-fold cross validation.
For hematological parameters, the optimal cutoffs to predict efficacy were determined using X-tile[1]. And multivariate Cox regression was conducted to identify independent biomarkers.
The clinical efficacy data cutoff date was June 13, 2022.
Results
Serum Proteomics
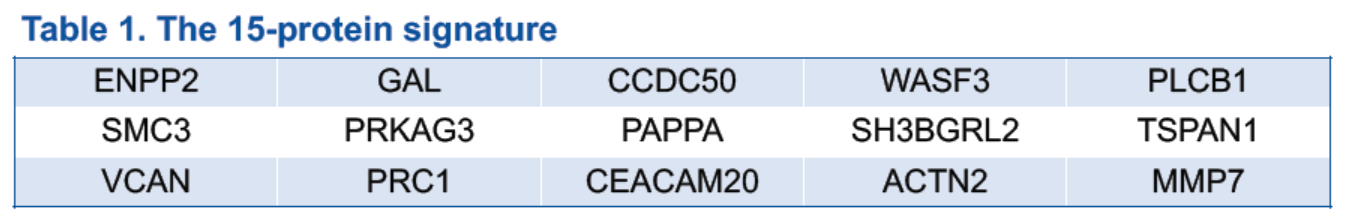
Proteomic profiling results were obtained from 168 patients (128 in the serplulimab group and 40 in the placebo group). The proteomic profiles were compared between responders and non-responders in the serplulimab group, and 181 DEP were identified. To further select the protein signature, investigators divided the patients into a training set (80 in the serplulimab group) and a validation set (48 in the serplulimab group and 40 in the placebo group). A generalized linear model selection with 5-fold validation was applied on the training set use 181 DEP and 15-protein signature was selected, which can sufficiently predict the responders from non-responders in the serplulimab group.
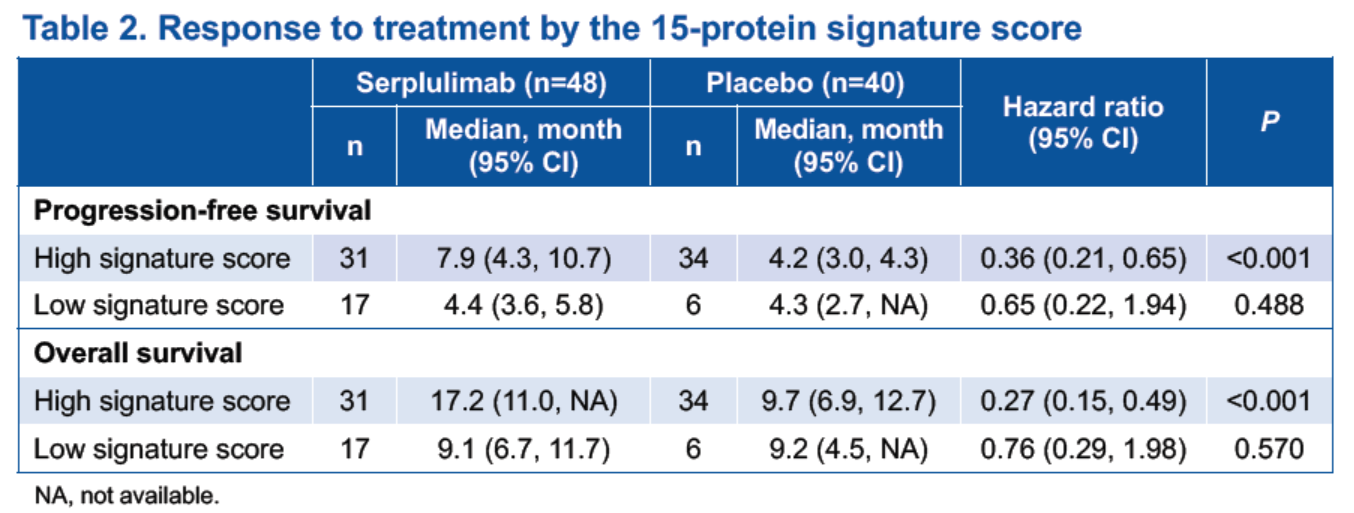
The predictive model based on the 15-protein signature showed an AUC of 0.982 in the training set, and 0.918 and 0.545 in the validation sets for the serplulimab and the placebo groups, respectively. Patients with high signature scores, which were generated from the protein set analysis of the 15-protein signature, derived benefits from adding serplulimab to chemotherapy in terms of PFS and OS (Table 2 and Figure 2), while those with low signature scores did not appear to derive benefits.
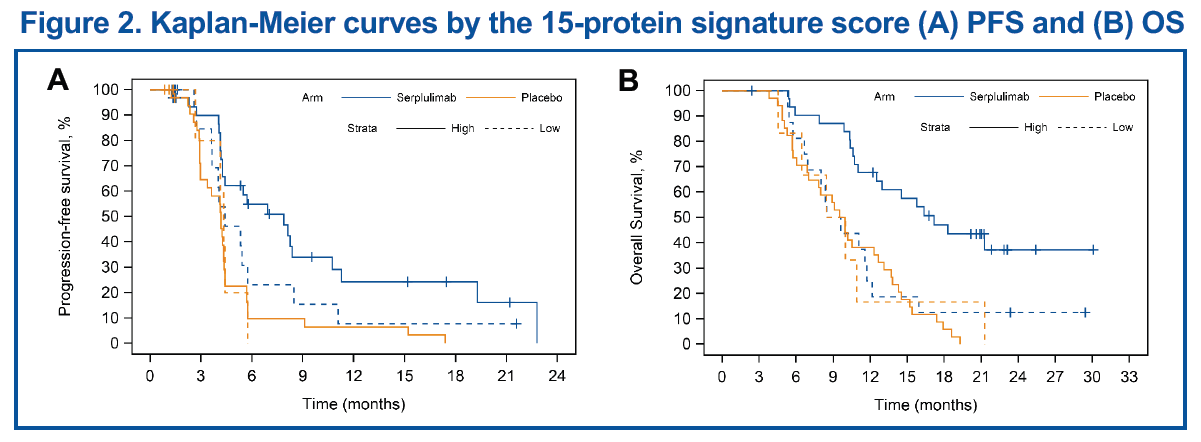
Genomic Mutations
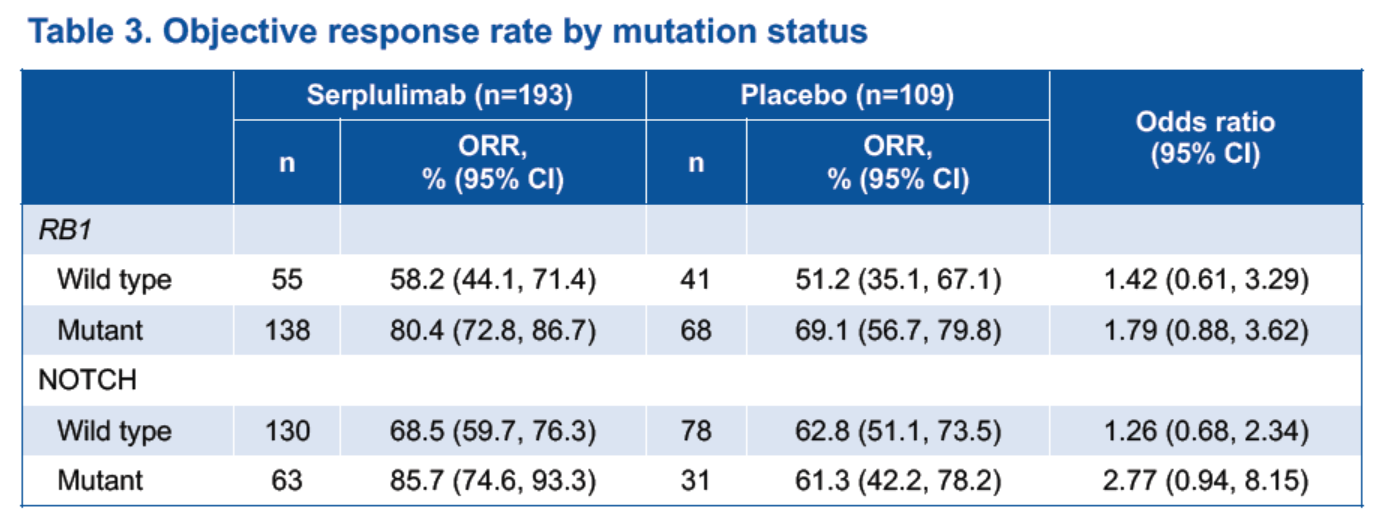
Genetic mutation data were available for 305 patients. After excluding 3 outliers, 302 patients were included in the analysis. Patients with mutations in the RB1 gene or the NOTCH pathway had higher response rates to serplulimab-chemotherapy compared with those without mutations (Table 3). While Patients with RB1 mutations and treated with serplulimab-chemotherapy had a tendency towards longer PFS and OS (Figure 3).
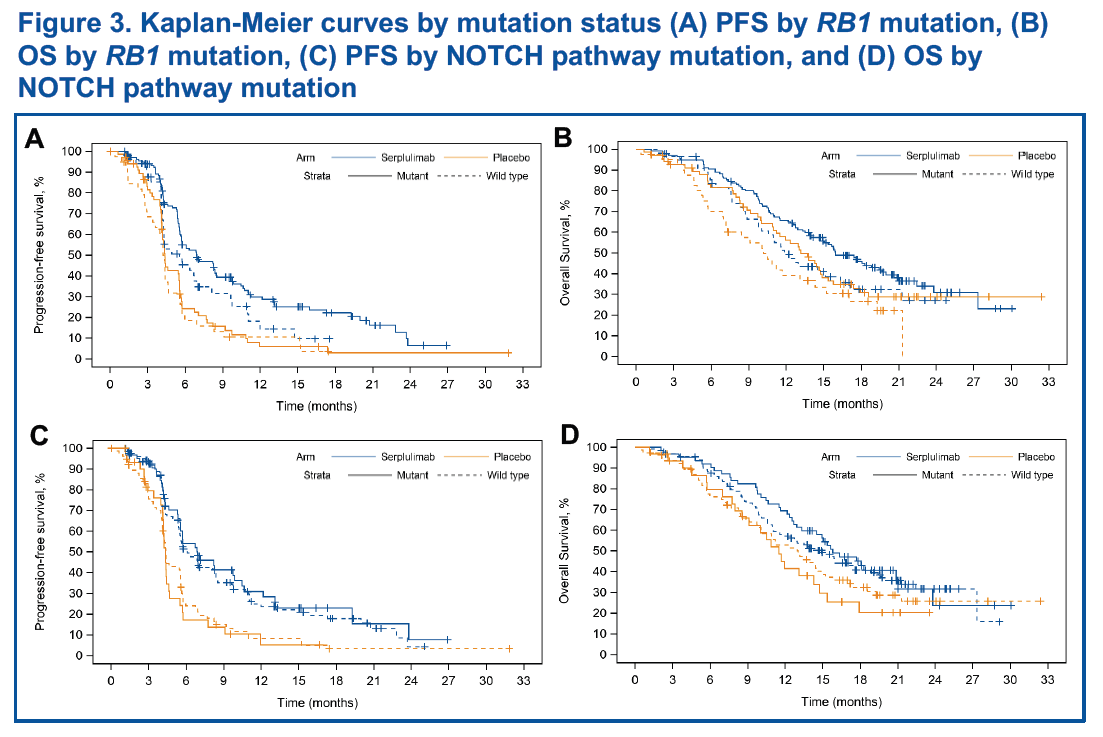
Hematological Parameters
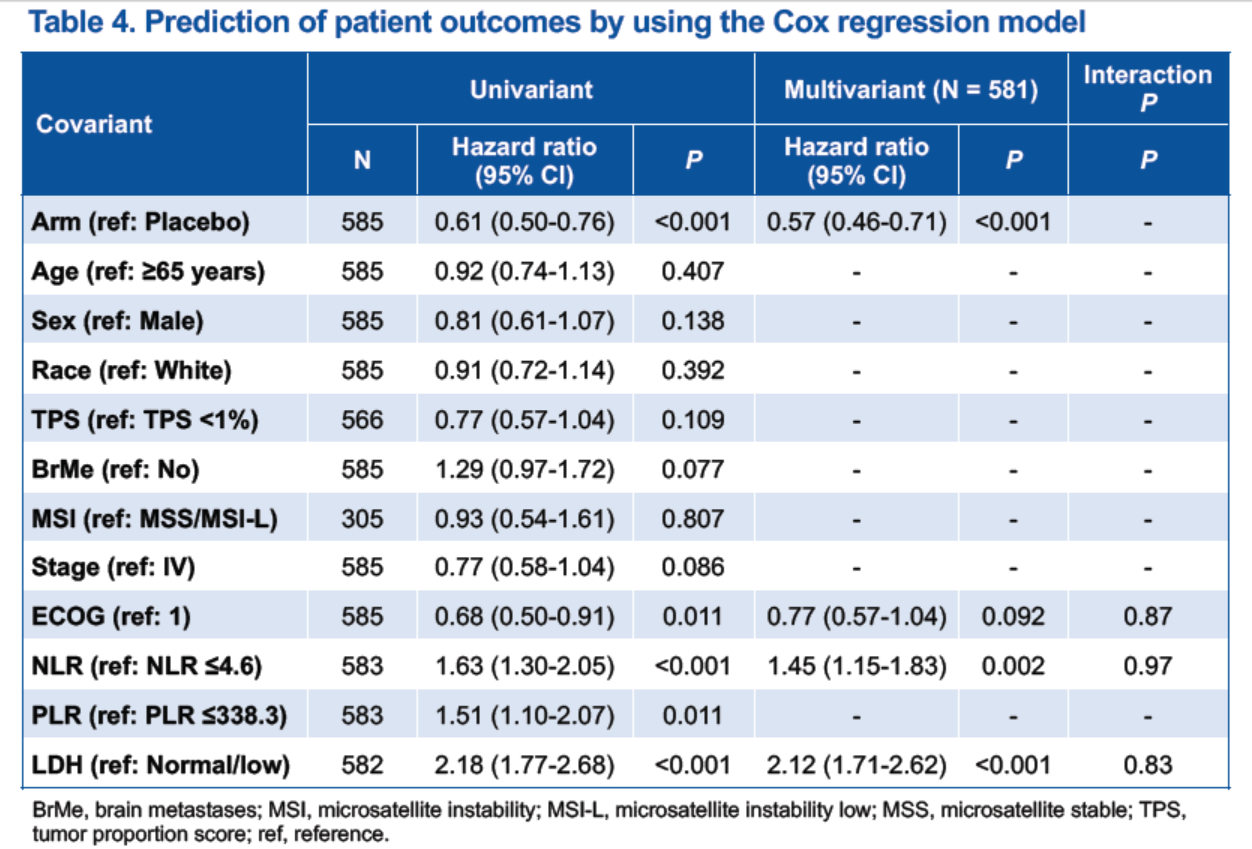
Among all 585 patients, 583 had available test results for NLR and PLR, while 582 had available test results for LDH. The analysis results indicated that high levels of baseline NLR, PLR, and LDH level were correlated with shorter PFS and OS in both treatment groups (Figure 4), implicating these are potential prognostic biomarkers for SCLC and correlate with poor patient prognosis.
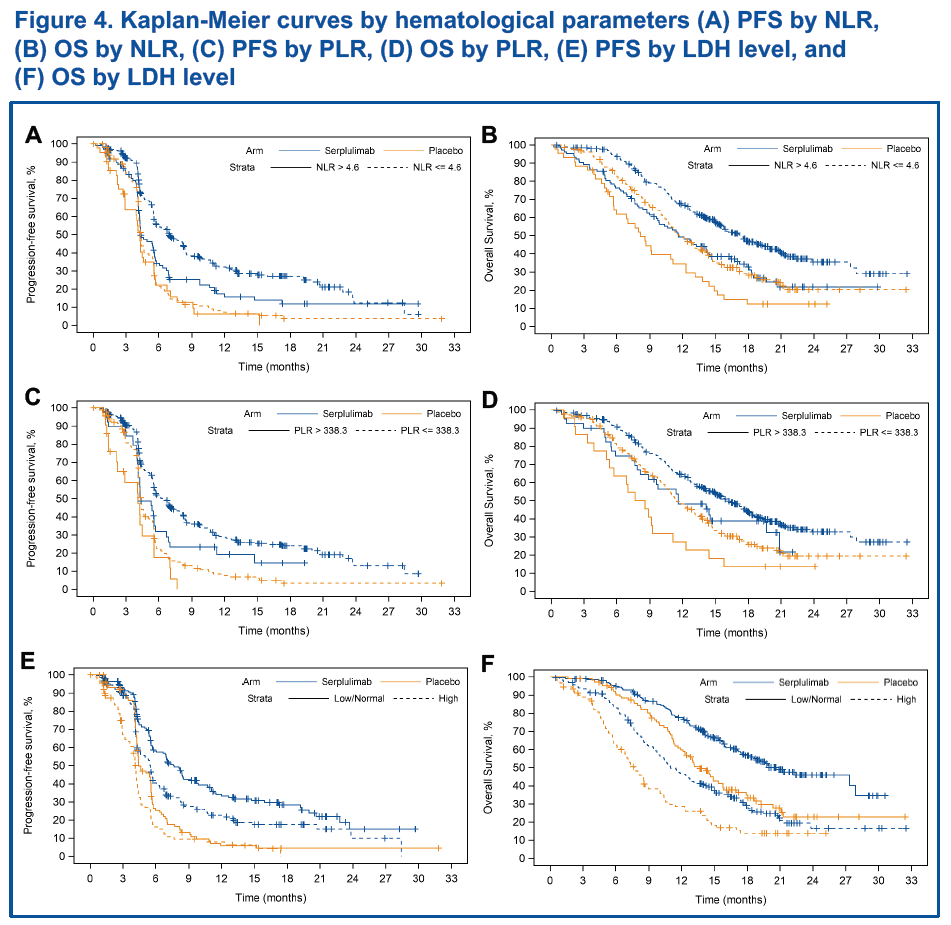
In the univariate Cox regression model, NLR, PLR and LDH levels were associated with patient outcomes in SCLC, while the multivariate Cox regression model showed that baseline NLR and LDH level were independent prognostic biomarkers (Table 4).
Conclusion
Although several treatments of immunotherapy plus chemotherapy have been approved for the treatment of small cell lung cancer (SCLC), biomarkers to predict immunotherapy for SCLC are not well characterised. There is an urgent need to identify reliable biomarkers that can predict elaborate biological characteristics of the ES-SCLC patients who can benefit from the treatment, further improving survival in SCLC patients.
Our results showed that a 15-protein signature score exhibited potential as a predictive biomarker for the efficacy of serplulimab plus chemotherapy. Additionally, mutations in the RB1 gene and the NOTCH pathway were associated with a more favorable treatment response to serplulimab plus chemotherapy (due to the limited sample size, the above results need further validation in clinical studies). Furthermore, high baseline NLR and LDH correlate with poor prognosis, suggesting that baseline NLR and LDH were independent prognostic biomarkers for ES-SCLC. These findings shed light on the potential of new biomarkers for immunotherapy plus chemotherapy in patients with ES-SCLC, which warrant further investigation.
Reference
1. Camp R L, et al. Clin Cancer Res. 2004;10:7252–7259.
About HANSIZHUANG
HANSIZHUANG (recombinant humanized anti-PD-1 monoclonal antibody injection, generic name: serplulimab injection) is the first anti-PD-1 mAb for the first-line treatment of SCLC and has been approved in China and Indonesia. Up to date, 4 indications are approved for marketing, 2 marketing applications are under review in China and the EU, and more than 10 clinical trials are ongoing across the world.
HANSIZHUANG was approved in China in March 2022 and has been approved by the National Medicinal Products Administration (NMPA) for the treatment of MSI-H solid tumours, squamous non-small cell lung cancer (sqNSCLC), extensive-stage small cell lung cancer (ES-SCLC), and esophageal squamous cell carcinoma (ESCC). The marketing applications of the first-line treatment for non-squamous non-small cell lung cancer (nsNSCLC) and ES-SCLC are under review by the NMPA and the European Medicines Agency (EMA), respectively. Focus on lung and gastrointestinal cancer, the synergy of HANSIZHUANG with in-house products of the company and innovative therapies are being actively promoted. The company has initiated more than 10 clinical trials on immuno-oncology combination therapies in a wide variety of indications with more than 3,900 subjects enrolled in China, the U.S., Turkey, Poland, Georgia and other countries and regions. The results of 4 pivotal trials of HANSIZHUANG were published in the Journal of the American Medical Association (JAMA), Nature Medicine, Cancer Cell, and the British Journal of Cancer, respectively. Furthermore, HANSIZHUANG was respectively recommended by the CSCO Guidelines for Small Cell Lung Cancer, the CSCO Guidelines for Non-Small Cell Lung Cancer, the CSCO Guidelines for Esophageal Cancer, the CSCO Guidelines for Colorectal Cancer, the CSCO Clinical Practice Guidelines on Immune Checkpoint Inhibitor, the China Guidelines for Radiotherapy of Esophageal Cancer, and other definitive guides, providing valuable references for clinical diagnosis and treatment of tumours. On the other hand, serplulimab was granted orphan drug designations by the U.S. FDA and the EC for the treatment of SCLC, and its bridging head-to-head trial in the United States to compare HANSIZHUANG to standard of care atezolizumab (anti-PD-L1 mAb) for the first-line treatment of ES-SCLC is well under way.
